TYPE 2 DIABETES
PREDICTING COMPLICATIONS
Type 2 diabetes is the most common form of diabetes, accounting for more than 90% of cases. It usually manifests in adulthood, in people aged 40 and up. Unfortunately, this form of diabetes is also increasingly appearing in younger people, due in part to higher obesity rates.
Type 2 diabetes is generally characterized by:
- insulin resistance
- low insulin production
This causes hyperglycemia, a condition in which blood sugar exceeds normal levels.
In the long term, hyperglycemia can lead to certain complications, affecting the kidneys, brain, heart and blood vessels.
Type 1 diabetes, on the other hand, usually occurs in people under the age of 20. It affects less than 10% of people living with diabetes. This form of diabetes is also known as insulin-dependent diabetes or juvenile diabetes.

Source : www.diabete.qc.ca
You are a patient living with type 2 diabetes.
You and your physician are concerned about the risk of complications associated with type 2 diabetes.
An innovative, non-invasive, user-friendly test based on your DNA is now available to predict your risk of developing complications.
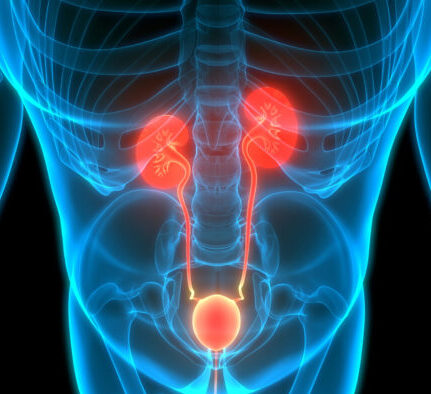
COMPLICATIONS RELATED TO TYPE 2 DIABETES
THAT CAN BE DETECTED BY THE TEST.
Kidney complication

Heart complication
Type 2 diabetes increases the risk of developing heart disease, which can lead to a heart attack.

Cardiovascular complication and stroke
Type 2 diabetes can also cause other complications such as retinopathy and neuropathy.
OPTITHERA
PREDICT TO PREVENT
OPTITHERA specializes in precision medicine as well as the development and clinical application of innovative technology. After many years of intensive research on type 2 diabetes, OPTITHERA developed a unique and effective test to predict the risk of kidney, heart and cerebrovascular complications related to type 2 diabetes.
Using a saliva sample that you can collect at a clinic or at home, OPTITHERA will analyze your genetic profile with the help of artificial intelligence to determine your risk of developing complications related to diabetes. We call this a polygenic risk score.
A polygenic risk score is based on your gender, age of onset, ethnicity, and data from your genetic code to determine your risk of developing complications related to type 2 diabetes before you even notice the first symptoms.
It’s important to identify the risks of complications as early as possible, as they can result in the damage of several organs. With your assessment results in hand, healthcare professionals (physicians and nurse practitioners) can establish a treatment plan for your condition.
Data privacy and medical ethics are a top priority for OPTITHERA. Rest assured that all information we collect remains strictly confidential.
ABOUT US
PREDICT TO PREVENT
so you can live better with type 2 diabetes—that’s OPTITHERA’s mission.
Our story
OPTITHERA was founded in 2016 by two distinguished professors at the University of Montreal, Pavel Hamet and Johanne Tremblay, and their collaborators. In recent years, OPTITHERA has analyzed data from more than 20,000 type 2 diabetes patients from 20 countries.
Based on these world-class clinical studies and with the help of artificial intelligence, researchers were able to develop a genome test that can inform your physician if you are at risk of developing complications related to type 2 diabetes.
Our team
News

A World-First in Genomics, a Saliva Test to Predict Complications Associated with Type 2 Diabetes, Powered by an Important Public-Private Partnership

A World-First in Genomics, a Saliva Test to Predict Complications Associated with Type 2 Diabetes, Powered by an Important Public-Private Partnership
OPTITHERA’s scientists contribute to shared data resources on prioritized genes of kidney function decline to help drug development pipelines.

OPTITHERA’s scientists contribute to shared data resources on prioritized genes of kidney function decline to help drug development pipelines.

OPTITHERA’s scientists contributed to the identification of diabetes-specific and non-diabetes specific genes of altered kidney function.

OPTITHERA’s scientists contributed to the identification of diabetes-specific and non-diabetes specific genes of altered kidney function.

OPTITHERA confirms that the introduction of its multiPRS test is cost effective in the care of renal complications of patients with diabetes

OPTITHERA confirms that the introduction of its multiPRS test is cost effective in the care of renal complications of patients with diabetes
Frequently Asked Questions
Contact us
Interested in learning more about the
OPTITHERA test?
Call us at 514 529-3374
or send us an email at
info@optithera.com
2815 Sherbrooke Street East, Suite 240
Montreal, Quebec, Canada H2K 1H2









